Chemistry Unit 1 Fundamentals of Chemistry
Chemistry chapter 1 Fundamentals of Chemistry on newsongoogle.com by Bilal Articles
Explore your academic success with NEWS ON GOOGLE! Discover the Chemistry chapter 1 Fundamentals of Chemistry curated by Bilal Articles. Prepare confidently for your exams with valuable insights and predictions. Visit newsongoogle.com for a strategic approach to academic excellence.
Major Concepts
1.1 Branches of Chemistry
1.2 Basic Definitions
1.3 Chemical species
1.4 Avogadro’ s Number and Mole
1.5 Chemical Calculations
Students Learning Outcomes
Students will be able to:
• Identify and provide examples of different branches of chemistry.
• Differentiate among branches of chemistry.
• Distinguish between matter and a substance.
• Define ions, molecular ions, formula units and free radicals.
• Define atomic number, atomic mass, atomic mass unit.
• Differentiate among elements, compounds and mixtures.
• Define relative atomic mass based on C-12 scale.
• Differentiate between empirical and molecular formula.
• Distinguish between atoms and ions.
• Differentiate between molecules and molecular ions.
• Distinguish between ion and free radicals.
• Classify the chemical species from given examples.
• Identify the representative particles of elements and compounds.
• Relate gram atomic mass, gram molecular mass and gram formula mass to
mole.
• Describe how Avogadro’s number is related to a mole of any substance.
• Distinguish among the terms gram atomic mass, gram molecular mass and
gram formula mass.
• Change atomic mass, molecular mass and formula mass into gram atomic
mass, gram molecular mass and gram formula mass.
BRANCHES OF CHEMISTRY
It is a fact that we live in the world of chemicals. We all depend upon different
living organisms which require water, oxygen or carbon dioxide for their survival.
Today chemistry has a wide scope in all aspects of life and is serving the humanity
day and night. Chemistry is divided into following main branches: physical
chemistry, organic chemistry, inorganic chemistry, biochemistry, industrial chemistry,
nuclear chemistry, environmental chemistry and analytical chemistry.
1.1.1 Physical Chemistry
Physical Chemistry is defined as the branch of chemistry that deals with the
relationship between the composition and physical properties of matter along with the
changes in them. The properties such as structure of atoms or formation of molecules
behavior of gases, liquids and solids and the study of the effect of temperature or
radiation on matter are studied under this branch.
1.1.2 Organic Chemistry
Organic Chemistry is the study of covalent compounds of carbon and hydrogen
(hydrocarbons) and their derivatives. Organic compounds occur naturally and are also
synthesized in the laboratories. Organic chemists determine the structure and properties
of these naturally occurring as well as synthesized compounds. Scope of this branch
covers petroleum, petrochemicals and pharmaceutical industries.
1.1.3 Inorganic Chemistry
Inorganic chemistry deals with the study of all elements and their compounds
except those of compounds of carbon and hydrogen (hydrocarbons) and their
derivatives. It has applications in every aspect of the chemical industry such as glass,
cement, ceramics and metallurgy (extraction of metals from ores).
1.1.4 Biochemistry
It is the branch of chemistry in which we study the structure, composition, and
chemical reactions of substances found in living organisms. It covers all chemical
processes taking place in living organisms, such as synthesis and metabolism of
biomolecules like carbohydrates, proteins and fats. Biochemistry emerged as a separate
discipline when scientists began to study how living things obtain energy from food or
how the fundamental biological changes occur during a disease. Examples of
applications of biochemistry are in the fields of medicine, food science and agriculture,
etc.
1.1.5 Industrial Chemistry
The branch of chemistry that deals with the manufacturing of chemical
compounds on commercial scale, is called industrial chemistry. It deals with the
manufacturing of basic chemicals such as oxygen, chlorine, ammonia, caustic soda,
nitric acid and sulphuric acid. These chemicals provide the raw materials for many other
industries such as fertilizers, soap, textiles, agricultural products, paints and paper, etc.
1.1.6 Nuclear Chemistry
Nuclear Chemistry is the branch of chemistry that deals with the radioactivity,
nuclear processes and properties. The main concern of this branch is with the atomic
energy and its uses in daily life. It also includes the study of the chemical effects resulting
from the absorption of radiation within animals, plants and other materials. It has vast
applications in medical treatment (radiotherapy), preservation of food and generation of
electrical power through nuclear reactors, etc.
1.1.7 Environmental Chemistry
It is the branch of chemistry in which we study about components of the
environment and the effects of human activities on the environment. Environmental
chemistry is related to other branches like biology, geology, ecology, soil and water. The
knowledge of chemical processes taking place in environment is necessary for its
improvement and protection against pollution.
1.1.8 Analytical Chemistry
Analytical chemistry is the branch of chemistry that deals with separation and
analysis of a sample to identify its components. The separation is carried out prior to
qualitative and quantitative analysis. Qualitative analysis provides the identity of a
substance (composition of chemical species). On the other hand, quantitative analysis
determines the amount of each component present in the sample. Hence, in this branch
different techniques and instruments used for analysis are studied. The scope of this
branch covers food, water, environmental and clinical analysis.
1.2 BASIC DEFINITIONS
Matter is simply defined as anything that has mass and occupies space. Our
bodies as well as all the things around us are examples of matter. In chemistry, we study
all types of matters that can exist in any of three physical states: solid, liquid or gas.
A piece of matter in pure form is termed as a substance. Every substance has a
fixed composition and specific properties or characteristics. Whereas, impure matter is
called a mixture; which can be homogeneous or heterogeneous in its composition.
We know that every substance has physical as well as chemical properties. The
properties those are associated with the physical state of the substance are called
physical properties like colour, smell, taste, hardness, shape of crystal, solubility,
melting or boiling points, etc. For example, when ice is heated, it melts to form water.
When water is further heated, it boils to give steam. In this entire process only the
physical states of water change whereas its chemical composition remains the same.
The chemical properties depend upon the composition of the substance. When a
substance undergoes a chemical change, its composition changes and a new substances
are formed. For example, decomposition of water is a chemical change as it produces
hydrogen and oxygen gases. All materials are either a substance or a mixture. Figure 1.1 shows simple classification of the matter into different forms.
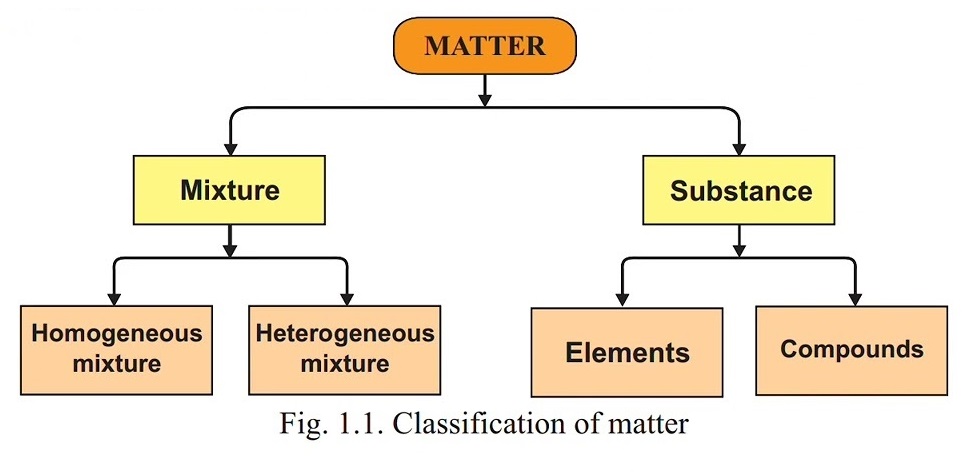
1.2.1 Elements, Compounds and Mixtures
1.2.1.1 Elements
In the early ages, only nine elements (carbon, gold, silver, tin, mercury, lead,
copper, iron and sulphur) were known. At that time, it was considered that elements were
the substances that could not be broken down into simpler units by ordinary chemical
processes. Until the end of nineteenth century, sixty-three elements had been discovered.
Now 118 elements have been discovered, out of which 92 are naturally occurring
elements. Modern definition of element is that it is a substance made up of same type of
atoms, having same atomic number and cannot be decomposed into simple substances
by ordinary chemical means. It means that each element is made up of unique type of
atoms that have very specific properties.
Elements occur in nature in free or combined form. All the naturally occurring
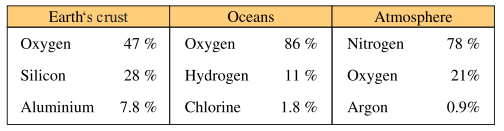
elements found in the world have different percentages in the earth’s crust, oceans and
atmosphere. Table 1.1. shows natural occurrence in percentage by weight of some major
elements around us. It shows concentrations of these major elements found in the three
main systems of our environment.
Table 1.1 Natural Occurrences by Weight % of Some Major Elements
Elements may be solids, liquids or gases. Majority of the elements exist as solids e.g. sodium, copper, zinc, gold, etc. There are very few elements which occur in liquid state e.g. mercury and bromine. A few elements exist as gases e.g. nitrogen, oxygen, chlorine and hydrogen.
On the basis of their properties, elements are divided into metals, non-metals and metalloids. About 80 percent of the elements are metals.
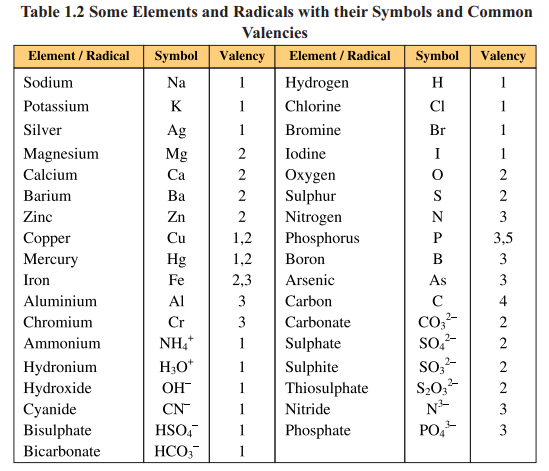
The unique property of an element is valency. It is combining capacity of an
element with other elements. It depends upon the number of electrons in the outermost
shell.
In simple covalent compounds, valency is the number of hydrogen atoms which
combine with one atom of that element or the number of bonds formed by one atom of
that element e.g. in the following compounds.
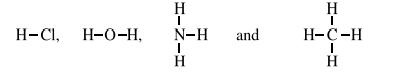
Compound:
Compound is a substance made up of two or more elements chemically combined
together in a fixed ratio by mass. As a result of this combination, elements lose their own
properties and produce new substances (compounds) that have entirely different
properties. Compounds can’t be broken down into its constituent elements by simple
physical methods. For example, carbon dioxide is formed when elements of carbon and
oxygen combine chemically in a fixed ratio.
Compounds can be classified as ionic or covalent. Ionic compounds do not exist
in independent molecular form. They form a three dimensional crystal lattice, in which
each ion is surrounded by oppositely charged ions. These oppositely charged ions attract
each other very strongly, as a result ionic compounds have high melting and boiling
points. These compounds are represented by formula units e.g. NaCl, KBr, CuSO .
The covalent compounds mostly exist in molecular form. A molecule is a true representative of the covalent compound and its formula is called molecular formula e.g. H O, HC1, H SO , Ch .
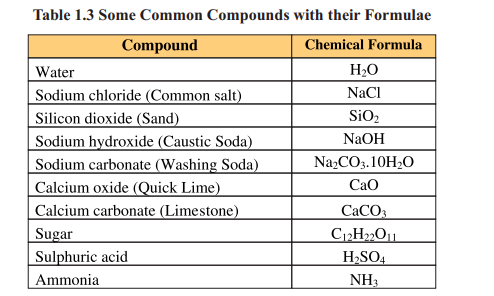
Mixture
When two or more elements or compounds mix up physically without any fixed ratio, they form a mixture. On mixing up, the component substances retain their own chemical identities and properties. The mixture can be separated into parent components by physical methods such as distillation, filtration, evaporation, crystallisation or magnetization. Mixtures that have uniform composition throughout are called homogeneous mixtures e.g. air, gasoline, ice cream. Whereas, heterogeneous mixtures are those in which composition is not uniform throughout e.g. soil, rock and wood.
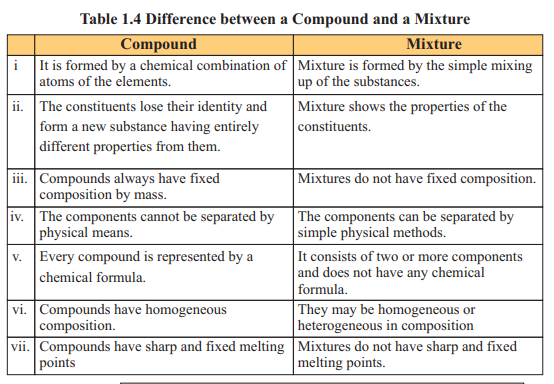
Atomic Number and Mass Number
The atomic number of an element is equal to the number of protons present in the nucleus of its atoms. It is represented by symbol ‘Z’ . As all atoms of an element have the same number of protons in their nuclei, they have the same atomic number.
Hence, each element has a specific atomic number termed as its identification
number. For example, all hydrogen atoms have 1 proton, their atomic number is Z=l. All
atoms in carbon have 6 protons, their atomic number is Z=6. Similarly, in oxygen all
atoms have 8 protons having atomic number Z=8 and sulphur having 16 protons shows
atomic number Z = 16.
The mass number is the sum of number of protons and neutrons present in the
nucleus of an atom. It is represented by symbol ‘A’.
It is calculated as A=Z+n where n is the number of neutrons.
Each proton and neutron has lamu mass. For example, hydrogen atom has one
proton and no neutron in its nucleus, its mass number A=l+0 =1. Carbon atom has 6
protons and 6 neutrons, hence its mass number A=12. Atomic numbers and mass
numbers of a few elements are given in Table 1.5
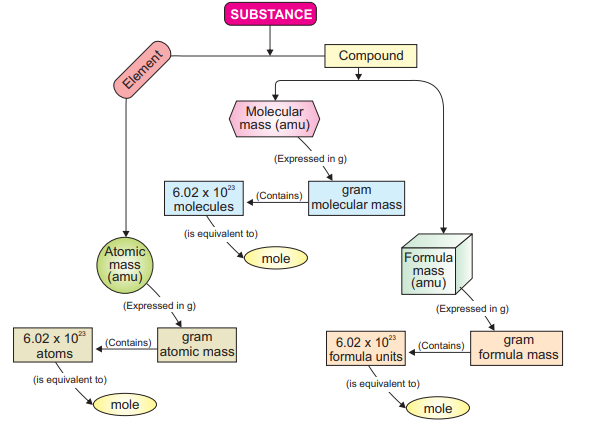
1.5.2 Mole (Chemist secret unit)
A mole is defined as the amount(mass) of a substance that contains 6.02 * l0
number of particles (atoms, molecules or formula units). It establishes a link between
mass of a substance and number of particles as shown in summary of molar calculations.
It is abbreviated as ‘mol’.
You know that a substance may be an element or compound (molecular or ionic).
Mass of a substance is either one of the following: atomic mass, molecular mass or
formula mass. These masses are expressed in atomic mass units (amu). But when these
masses are expressed in grams, they are called as molar masses.
Scientists have agreed that Avogadro’s number of particles are present in one molar
mass of a substance. Thus, quantitative definition of mole is the atomic mass, molecular
mass or formula mass of a substance expressed in grams is called mole.
For example:
Atomic mass of carbon expressed as 12 g = 1 mol of carbon
Molecular mass of H O expressed as 18 g = 1 mol of water 2
Molecular mass of H SO expressed as 98 g = 1 mol of H SO 2 4 2 4
Formula mass of NaCl expressed as 58.5 g = 1 mol of NaCl
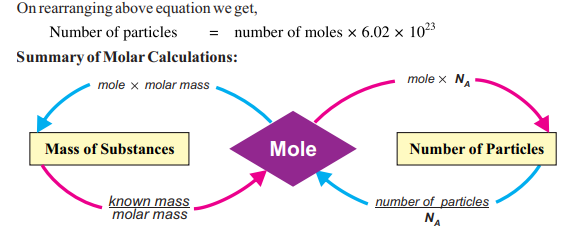
Thus, the relationship between mole and mass can be expressed as:
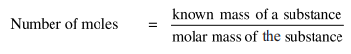
Or,
Mass of substance (g) = number of moles x molar mass
A detailed relationship between a substance and a mole through molar mass and
number of particles is presented here.
Summary showing a relationship between a substance and a mole.
1.6.2 Mole-Particle Calculations
In these calculations, we can calculate the number of moles of a substance from the given number of particles. (These particles are the atoms, molecules or formula units).
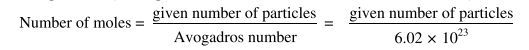
Long Answer Questions.
1. Define element and classify the elements with examples.
2. List five characteristics by which compounds can be distinguished from
mixtures.
3. Differentiate between the following with examples:
i. Molecule and gram molecule
ii. Atom and gram atom
iii. Molecular mass and molar mass iv. Chemical formula and
gram formula
4. Mole is SI unit for the amount of a substance. Define it with examples?
Keep Visiting newsongoogle.com by Bilal Articles
Related posts:
- Class 9th chemistry important questions
- Class 9th chemistry guess paper
Related keywords:
- Chemistry unit 1 all notes pdf free download
- Chemistry unit 1 all notes pdf download
- Chemistry unit 1 all notes pdf
- 1st year chemistry chapter 1 notes
- chemistry chapter 1 notes class 9
- 1st year chemistry notes pdf download
- class 9 chemistry notes chapter 1 pdf
- 1st year chemistry notes chapter 1 stoichiometry
